Background:
Patients with hematologic malignancies (HM) receive more aggressive care at the end of life (EOL) and have suboptimal hospice utilization when compared to patients with solid tumors. One reason for this is a high rate of transfusion dependence (TD). Many hospice agencies decline to provide palliative transfusions because of cost, philosophical objections, and/or logistical barriers, and as a result, many TD patients delay hospice enrollment or never enroll at all. In response to a call from ASH for novel care models to address this issue, we designed a pilot study of an open access transfusion model in hospice care. Our hypothesis was that the provision of transfusions for patients with HM on hospice would be feasible and would result in timely hospice referral and less aggressive EOL care.
Methods:
We conducted a single-center, prospective pilot study [NCT05063591] enrolling adults with aggressive HM who were TD and hospice-eligible. We concurrently enrolled patients' primary caregivers. Patients were evaluated weekly during a clinic visit or telephone call to assess potential symptoms related to anemia or thrombocytopenia. Relevant symptoms triggered a CBC assessment, and, if appropriate, administration of a transfusion of red blood cells (RBCs) and/or platelets.
Caregivers completed the Caregiver Evaluation of Quality of EOL Care survey (CEQUEL, Higgins et al. PLoS One, 2013) ≥1 month after the patient death, to assess perception of the patient's EOL care.
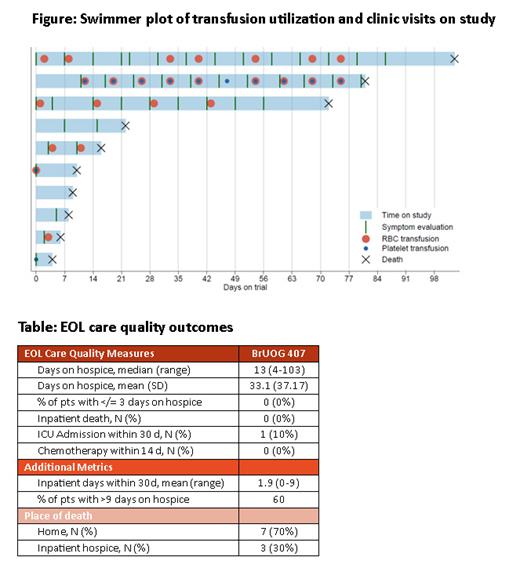
The primary outcome was feasibility of the care model, defined as 70% rate of successful enrollment after screening. Secondary outcomes included standard EOL care quality measures: hospice length of stay (LOS), inpatient days and ICU days in the last 30 days of life, inpatient deaths, and the use of chemotherapy in the last 14 days of life. Exploratory outcomes included caregiver's assessment of EOL care quality.
Results:
Of 22 patients and 9 caregivers screened, 10 patients (45%) and 8 caregivers enrolled. 4 screened patients died before consenting. The median patient age was 80 (range, 69-90). Most patients (90%) had a myeloid malignancy (AML, MDS, or CMML). The mean number of transfusions on study was 2.4 units of RBCs (range, 0-9) and 1.2 units of platelets (range, 0-10) ( Figure). The most common transfusion-triggering symptom was fatigue (N=40 transfusion events), followed by shortness of breath (N=15). The median LOS on hospice was 13 days (range, 4-103; Table). 60% of patients spent >9 days on hospice, and no patient spent ≤ 3 days on hospice. All enrolled patients died outside of the hospital: 60% at home and 40% at inpatient hospice. No patient received chemotherapy within 14 days of death, and 1 (10%) was admitted to the ICU within 30 days of death (prior to trial enrollment). There were no disenrollments due to transfusion reactions. One patient (10%) revoked hospice for hospitalization, but re-enrolled and died in inpatient hospice.
When caregivers evaluated their loved one's EOL care, 100% reported that the patient experienced a peaceful death; 100% felt that medical intervention did not result in increased suffering; 86% agreed that EOL was not prolonged by medical intervention longer than they would have wished, and 86% felt that the patient suffered much less than expected. The mean CEQUEL score was 24 (range, 20-26, higher scores indicating a better perception of EOL care).
Conclusion:
Providing blood transfusions to patients with HM on hospice was feasible in our symptom-driven care model. The enrollment rate was short of the 70% goal, but some screened patients died before enrolling, suggesting that hospice was considered too late in the disease course, and that additional factors beyond TD may contribute to suboptimal hospice utilization among these patients.
When compared with our population-based study describing EOL outcomes of patients with HM (Egan et al., Blood Adv. 2020), our current outcomes compare favorably, with longer median time on hospice (13 vs 9 days), fewer hospital deaths (0 vs 27%), and less chemotherapy at the EOL (0 vs 13%). Importantly, caregiver perception of EOL care quality was favorable.
Our findings illustrate the potential of palliative blood transfusions to improve the EOL care quality for HM patients on hospice, and highlight the need for earlier hospice referral while pursuing care models that address the specific needs of this unique TD population.
Disclosures
Reagan:Pfizer: Research Funding; Rigel: Membership on an entity's Board of Directors or advisory committees. Olszewski:Genmab, Blue Cross/Blue Shield of Rhode Island, Schrodinger, ADC Therapeutics, BeiGene: Consultancy; Leukemia & Lymphoma Society, Genetech, Inc. / F. Hoffmann-La Roche Ltd, Adaptive Biotechnologies, Precision Biosciences, Genmab: Research Funding. LeBlanc:Dosentrx: Current equity holder in private company; UpToDate: Patents & Royalties; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Agilix: Consultancy, Honoraria; American Cancer Society: Research Funding; Deverra Therapeutics: Research Funding; Duke University: Research Funding; Incyte: Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Meter Health: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; Flatiron: Consultancy, Honoraria; CareVive: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; BlueNote: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Speakers Bureau; Agios: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Research Funding; Leukemia and Lymphoma Society: Research Funding; National Institute of Nursing Research/National Institutes of Health: Research Funding; Seattle Genetics: Research Funding; Servier: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal